Imagine there’s an inexpensive way to purify wastewater that does not involve the use of chemicals? Well, researchers at MIT have now developed a new process to remove pollutants from water, which could have far-reaching impact by making wastewater treatment more affordable and, therefore, more accessible worldwide.
The waste of wastewater
The theme for this year’s World Water Day was wastewater with a call to action to “reduce and reuse” wastewater globally. Why wastewater?
Because over 80 percent of the wastewater in the world goes right back into the ecosystem without it being treated or reused. That’s a waste of a precious resource with a double whammy on the environment. First, untreated water pollutes the environment, but 1.8 billion people today only have contaminated water as their source of drinking water. Second, water is a finite source, and we already know that groundwater, the largest source of freshwater, is at risk of being depleted in many parts of the world by 2050.
The potential of wastewater as a valuable resource is enshrined in Sustainable Development Goal (SDG) 6.3, which aims to “improve water quality by reducing pollution, eliminating dumping and minimizing release of hazardous chemicals and materials, halving the proportion of untreated wastewater and substantially increasing recycling and safe reuse globally” by 2030.
The possibility of achieving the SDG 6.3 goal has just been strengthened by MIT researchers who have invented a new method that can remove “even extremely low levels of unwanted compounds” from water in a sustainable way.
The new method provides the world with an inexpensive and eco-friendly alternative to remove pollutants from water. Up until now, the prevailing methods are energy- and chemical-intensive, which are not just expensive but also unhealthy for the environment.
The team behind the invention is MIT postdoc student Xiao Su, MIT’s Ralph Landau Professor of Chemical Engineering T. Alan Hatton, and five other researchers at MIT and at the Technical University of Darmstadt in Germany.
According to Hatton, the new system works at low voltages and pressures so it doesn’t have the downsides of other conventional systems that require high voltages and pressures. He also added that the new system doesn’t need the addition of chemicals to release captured pollutants and regenerate adsorbents. Instead, in the new system, one could “just flip a switch” to achieve the same result by switching the polarity of the electrodes.
How does the new system work?
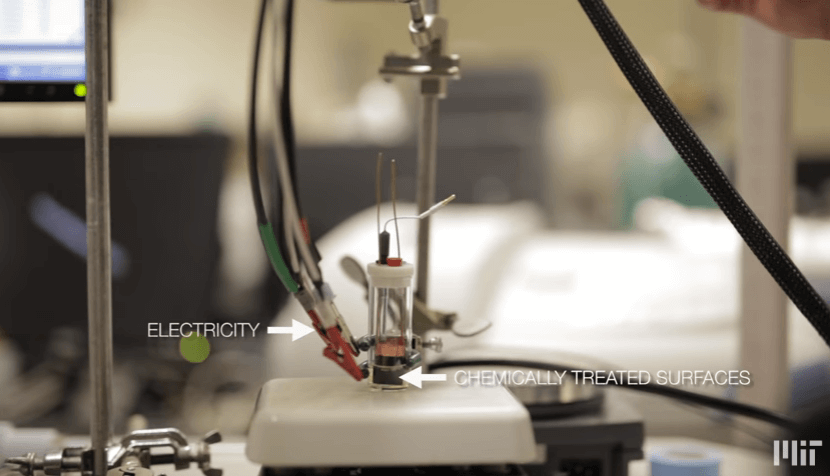
The new system uses electricity and chemically treated surfaces to selectively remove organic contaminants like pesticides, chemical waste products, and pharmaceuticals from water, even when these contaminants are present in small but still dangerous concentrations. It also addresses problems inherent in current conventional systems, including fluctuations in acidity and losses in performance stemming from competing surface reactions.
Current systems for removing dilute concentrations of contaminants from water are not just expensive but also less effective when it comes to low concentrations. They also often require high voltages that tend to produce contaminating compounds, a side reaction, and are hindered by excess background salts.
In contrast, in MIT’s new system, the water flows between chemically treated, or “functionalized,” surfaces that serve as positive and negative electrodes. These electrode surfaces are coated with Faradaic materials, which are materials that can undergo reactions and become positively or negatively charged. Then an electric source is added. As water flows between these chemically treated electrodes, the surface materials can be adjusted to bind strongly with a specific type of pollutant molecule, which the team demonstrated with ibuprofen and various pesticides.
The researchers found that this process is effective even when removing such pollutant molecules at parts-per-million concentrations.

The researchers also found that the use of appropriately functionalized electrodes, arranged asymmetrically, resulted in almost completely eliminating the side reactions present in other systems. It also makes it possible to selectively, and simultaneously, remove both positive and negative toxic ions at the same time, which the team demonstrated with two herbicides, paraquat and quinchlorac.
Su explained that the same selective process could be used to recover high-value compounds in a chemical or pharmaceutical production plant, where they might otherwise be wasted.
“The system could be used for environmental remediation, for toxic organic chemical removal, or in a chemical plant to recover value-added products, as they would all rely on the same principle to pull out the minority ion from a complex multi-ion system,” Su said in a statement.
While the system is inherently highly selective, Su believes that in practice it would involve a multi-step approach to tackle a variety of compounds in sequence, which would depend on the exact application.
“Such systems might ultimately be useful for water purification systems for remote areas in the developing world, where pollution from pesticides, dyes, and other chemicals are often an issue in the water supply,” he said in a statement. “The highly efficient, electrically operated system could run on power from solar panels in rural areas for example.”
MIT’s new system is described in the journal Energy and Environmental Science.
The research team has been recognized for the ongoing development of water treatment technology, including grants from the J-WAFS Solutions and Massachusetts Clean Energy Catalyst competitions, and the researchers also won last year’s MIT Water Innovation Prize.
The researchers have applied for a patent, and intend to scale up their prototype devices in the lab and improve the chemical robustness.
“We definitely want to implement this in the real world,” Hatton said in a statement.
What’s next for the new system?
TUN spoke with Hatton and Su to follow up on their plans for their new system.
We were informed that the technology hasn’t been tested outside of the lab yet, but that they are beginning to work with real effluent waters. They hope to proceed to field study in the next six to twelve months.
Both of them are positive about what the new system can achieve.
“This technology shows promise for the remediation of contaminated water resources over a wide range of scales, from household water purification units to large industrial and municipal water treatment facilities, at lower costs and pressures than many other approaches, and with greater selectivity for micropollutants of direct concern to the EPA [Environmental Protection Agency],” said Hatton.
The technology also has other potential applications.
“It is a versatile technology with a wide range of applications, not only in water treatment, but also in biological applications, and in the mitigation of greenhouse gas emissions,” said Hatton.
Su agrees that the new system would have an impact in the real world.
“As Alan [Hatton] mentioned, something we are very interested currently is scaling up our technologies and testing them in real practical conditions,” said Su.
“We expect our work, once implemented, to have an impact on water purification both here in the U.S., as well as worldwide, especially in developing countries which are facing serious pollution problems.”
The new system is ideal for rural and remote areas, where many lack access to safe water. A recent study in Australia, for instance, found that unclean water supply could contribute to a lower life span in the country’s rural and remote areas.
In addition to the industrial applications, one advantage of our electrochemical platform is that it can be deployed on-site, in the field in rural and remote areas. Our water purification device can then be integrated with solar or other renewable sources, and will hopefully attend the need of small communities.
[divider]
RELATED ARTICLES:
Universities Help to Alleviate Water Crisis
New Device from MIT and UC Berkeley Pulls Water from Dry Air