As climate change, pollution and population levels are on the rise, humanity is at risk of depleting its most necessary resource — freshwater.
The issue is so severe that by 2025, 1.8 billion people are expected to live in areas with absolute water scarcity, and ⅔ of the world could be under water-stressed conditions, the United Nations warns.
Water scarcity can cause widespread death and disease, and lead to the displacement of hundreds of millions of people worldwide.
In this article, we highlight recent efforts by several universities to tackle the global water crisis.
What is water scarcity?
Water scarcity can occur due to physical shortage, failure of institutions to ensure a regular supply, or a lack of adequate infrastructure.
The UN reported that water use has grown at more than twice the rate of population increase over the last century, which has caused an increasing number of regions to face difficulty obtaining water from reliable sources.
Since freshwater only makes up about 2.5 percent of the Earth’s water supply, areas around the world are struggling to keep up with population demands.
There are currently 844 million people living without access to clean water, which causes more deaths per year than violence or war.
Where do we get our water?
Of the 2.5 percent of freshwater available for human consumption, only about 1 percent of it is easily accessible, as much of it is trapped in glaciers and snowfields.
Groundwater is the largest source of freshwater in the world, and supplies drinking water to over 50 percent of the U.S. population.
Unfortunately, due to the overpumping of aquifers, groundwater is being rapidly depleted in many parts of the world, including the U.S. Aquifers are underground layers of water-bearing permeable rock or loose materials, such as gravel, sand or silt, from which groundwater can be extracted using a water well.
Groundwater is being depleted so fast that aquifers in India, southern Spain and Italy could be depleted between 2040 and 2060, while those in California’s Central Valley, Tulare Basin and southern San Joaquin Valley could be depleted sooner than that.
Additionally, in the U.S., major freshwater sources including the Colorado River and Lake Mead in Arizona are beginning to dry up.
According to The Water Project, some researchers believe that Lake Mead, which supplies water to 22 million people in the U.S., could be dry by 2021.
University’s efforts to relieve the water crisis
Though the numbers can be discouraging, there have been numerous efforts by universities to help relieve the water crisis.
From filtration to desalination techniques, here are a few examples of the exceptional work that universities have recently achieved.
MIT, utilizing brackish groundwater
According to MIT researchers, one way to help solve the water crisis is by looking beyond freshwater.
The researchers have found a way to desalinate various compositions of brackish groundwater — a type of groundwater that contains more salinity than freshwater, but not as much as seawater.
The amount of brackish groundwater in the U.S. is about 800 times greater than the total amount of groundwater withdrawn nationwide, yet less than 1 percent of the nation’s water supply comes from this source.
So, according to the researchers, using even a fraction of the abundant brackish water in the U.S. could dramatically improve the prospects for water-starved communities.
“The U.S. has more brackish groundwater than fresh groundwater. And much of the world’s fresh groundwater is being heavily used, to the point that many fresh water aquifers are showing signs of decline,” said John Lienhard, director of the Abdul Latif Jameel World Water and Food Security Lab at MIT.
Lienhard and his team used thermodynamics, a science that deals with the relationship between heat and energy, to study how different compositions of brackish groundwater would affect the energy needed for desalination.
By doing this, the researchers were able to formulate a large dataset of information on the processes and requirements to desalinate brackish groundwater, as well a map of areas around the U.S. and world that have potentially usable brackish groundwater sources.
“Growing population and a warming and more variable climate poses unprecedented challenges for our cities, farms, and industry,” said Lienhard.
“Sustainable use of brackish groundwater, through desalination, can help expand our freshwater supplies. This approach can be one component of an integrated water management strategy for the many parts of the world threatened by water scarcity.”
CMU, plant-based mechanisms for water filtration
Another method that could help water-starved areas, particularly in sub-tropical, or tropical regions such as India, is by using plant materials as a means to filter unclean water.
Researchers at Carnegie Mellon University have developed a way to combine seed proteins from the Moringa Oleifera plant, a commercially valuable plant native to India, with sand filtration techniques to produce a simple and effective filtration mechanism.
The resulting medium is called “f-sand” and works by using opposite electrical charges.
“Sand is mostly negatively charged. The proteins from the Moringa oleifera seeds are positively charged, and most of the suspended contaminants (suspended clay or other mineral particles, bacteria, decomposing organic material naturally found in the environment) that should be removed from water to make it potable are negatively charged,” said Bob Tilton, the Chevron Professor of Chemical Engineering and Biomedical Engineering at CMU.
To prepare f-sand, Tilton explained, the water-soluble proteins are first extracted from the seeds by crushing them and soaking them in water. The resulting solution is then poured into a very thick, wet mixture of sand and water called a slurry.
At this time, the negative sand charge attracts to the positive protein charge, causing the proteins to stick to the sand, and subsequently, create “f-sand.” The excess water can then be drained out and the f-sand can be poured into a column
Then, to use the f-sand, one would simply pour water through the column, and the negatively charged contaminants would stick to the positively charged proteins on the sand grains, creating a simple and resourceful filtration system.
Tilton explains that f-sand will be easily adapted by local users, since the technique requires few tools and fairly simple work.
“Systems could be set up to work at a very localized level, probably even at the individual household level,” said Tilton.
UT Dallas/Penn State, water-harvesting technology inspired by beetles
In yet another creative way to help relieve our growing water crisis, researchers from the University of Texas at Dallas and Penn State University have developed a surface that can rapidly collect water molecules from fog and air vapor and direct them toward a reservoir along lubricated microgrooves.
The “hydrophilic directional slippery surface,” or SRS, was inspired by the process in which living organisms, such as desert beetles, pitcher plants and rice leaves, naturally collect and direct water.
“Desert beetles used hydrophilic bumps to absorb water droplets from fogs, and also used patterned hydrophobic waxes to remove those droplets for drinking,” said Simon Dai, an assistant professor of mechanical engineering in the Erik Jonsson School of Engineering and Computer Science at UT Dallas.
While the hydrophilic bumps on desert beetles demonstrate how to collect and combine water droplets from the air, the slippery surfaces of pitcher plants and the miniscule directional grooves on rice leaves demonstrate how to quickly direct the water droplets toward a reservoir.
The combination of these natural designs creates a surface capable of both capturing and directing water droplets efficiently.
To create the water-harvesting technology, the researchers constructed a surface and etched directional, nano-sized structures into it. They then coated the surface with a hydrophilic liquid lubricant.
The lubricant serves a dual purpose by attracting water and creating a slippery surface, so the water droplets that form can easily move down and combine to make larger droplets.
“We infused our directional rough surfaces with hydrophilic liquid lubricant that is molecularly mobile, so it can help the collected water coalesce into larger drops,” Dai said in a statement.
“Furthermore, the material is scalable. Unlike the beetle-inspired surfaces where water droplet creation only occurs in specific areas, we can create small or large surfaces, all with the ability to trap and move water quickly everywhere on the surface.”
The combination of directional grooves and the hydrophilic slippery surface proved to be more effective at capturing and directing water droplets than comparable surfaces.
Hydrophilic, or water-attracting, surfaces like the SRS can be used for a number of purposes and industries, such as air conditioning, dropwise condensation for power generation and desalination, and water harvesting in arid regions.
“Existing processes for creating fresh water, such as desalination, rely on the transition from vapor to water,” Dai said in a statement.
“We wanted to create a surface that can both capture and direct water droplets efficiently.”
Rice University, sequestering toxins from water
Rice University researchers have been working on a treatment system that can selectively pull harmful toxins from drinking water and wastewater from factories, sewage systems and oil and gas wells.
The system uses a set of composite electrodes that enable capacitive deionization, a technology to deionize water by applying an electrical potential difference over two electrodes, which are often made of porous carbon.
The porous, charged electrodes are able to selectively pull target ions from fluids passing through a maze-like system. Then, when the pores get filled with toxins, the electrodes can be cleaned, restored and reused.
Led by Qilin Li, a professor of civil and environmental engineering and of materials science and nanoengineering, the Rice treatment system is expected to be a low-cost, energy saving alternative to traditional systems.
“Traditional methods to remove everything, such as reverse osmosis, are expensive and energy intensive,” Li said in a statement.
“If we figure out a way to just fish out these minor components, we can save a lot of energy.”
So far, the researchers have tested the system on the removal of sulfate ions, a scale-forming mineral that can give water a bitter taste and act as a laxative.
To do this, the system’s electrodes were coated with activated carbon, which was in turn coated by a thin film of tiny resin particles held together by quaternized polyvinyl alcohol.
When sulfate-contaminated water flowed through a channel between the charged electrodes, sulfate ions were attracted by the electrodes, passed through the resin coating and stuck to the carbon.
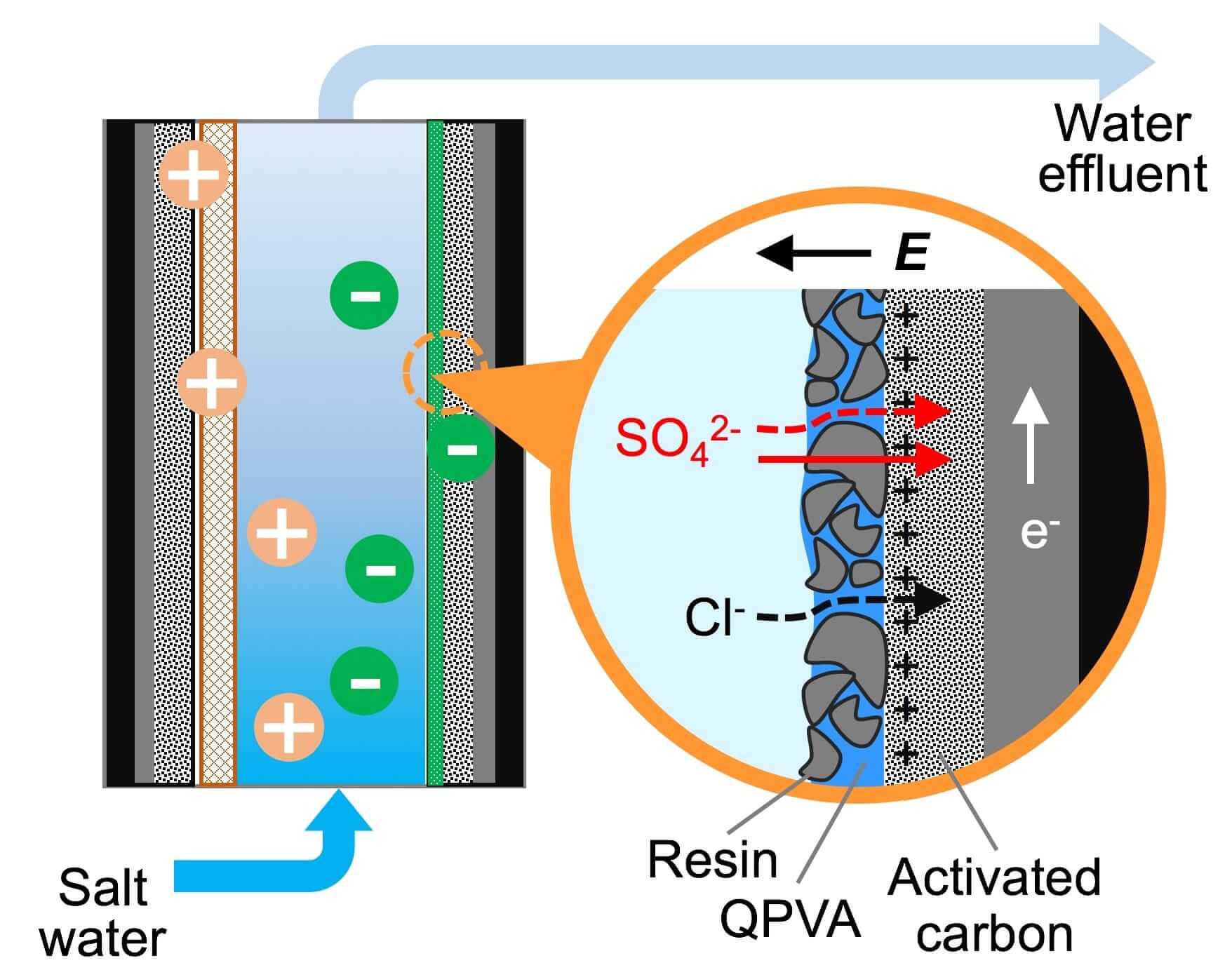
Tests in the lab showed that the positively charged coating captured sulfate ions over salt at a ratio of more than 20 to 1.
“This is part of a broad scope of research to figure out ways to selectively remove ionic contaminants,” Li said in a statement. “There are a lot of ions in water. Not everything is toxic. For example, sodium chloride (salt) is perfectly benign. We don’t have to remove it unless the concentration gets too high.”
“For many applications, we can leave non-hazardous ions behind, but there are certain ions that we need to remove. For example, in some drinking water wells, there’s arsenic. In our drinking water pipes, there could be lead or copper. And in industrial applications, there are calcium and sulfate ions that form scale, a buildup of mineral deposits that foul and clog pipes.”
The researchers are currently working on developing coatings for other contaminants and working with labs at the University of Texas at El Paso and Arizona State University on large-scale test systems.
Conclusion
The global water crisis affects everyone — even those who don’t feel the direct impact — and as depletion levels continue to rise, we must all do our part to ensure water security and cut back on water-related disease.
Fortunately, efforts are being initiated by scientists, activists and non-profit organizations to help relieve the crisis around the world.
In today’s interconnected age, we are in a unique position to work together to protect our most vital resource.
Now more than ever, people and organizations, such as Water.org, Drop in the Bucket, Water For People and Water is Life, are able to work on a global level to help water-starved communities and make a significant impact on the changing world.





