In 1949, Chris Polge won the Nobel Prize in Medicine for his ability to cryopreserve or freeze sperm cells. Since then, advancements in cryopreservation technology have led to the successful freezing and thawing of human embryos and eggs. This known technology also works for similar mammals and wildlife species. But until now, researchers have failed to cryopreserve fish embryos, due to their size.
In a recent study, researchers at the University of Minnesota and the Smithsonian Conservation Biology Institute (SCBI) have used new gold nanotechnology and lasers to cryopreserve embryos of zebrafish, whose genomes are similar to humans. The full research is available at scientific journal ACS Nano.
This new technology could lead to the repopulation of endangered fish species, support the growth of depleting coral reefs, and heighten our understanding of human disease through fish research models.
Traditionally, to effectively cryopreserve an embryo, scientists must cool the embryo to a stable state and heat it back up quicker than it was cooled. Scientists often inject cryoprotectants (antifreeze agents) into the embryo to prevent the growth of deadly ice crystals that form during the reheating process. Fish embryos are much larger than human embryos, so it generally takes a long time for them to thaw out. Embryonic membranes in fish are also mostly impenetrable, and often reject cryoprotectants.
This most recent study is successful due to new gold nanotechnology developed by John Bischof, associate director of the Institute for Engineering in Medicine at the University of Minnesota and senior author of the study.
Gold nanoparticles are tiny cylindric objects that convert absorbed light into heat.
“The new idea was to pair the tiny gold nanoparticles in with the anti-freeze agents in the microinjection,” Bischof explained to The University Network (TUN). “These gold particles are extremely small, biologically inert and very good at absorbing laser light of specific wavelengths. Most biological tissue is translucent, so we need these gold particles to absorb the heat from the laser and reradiate the heat to the embryo.”
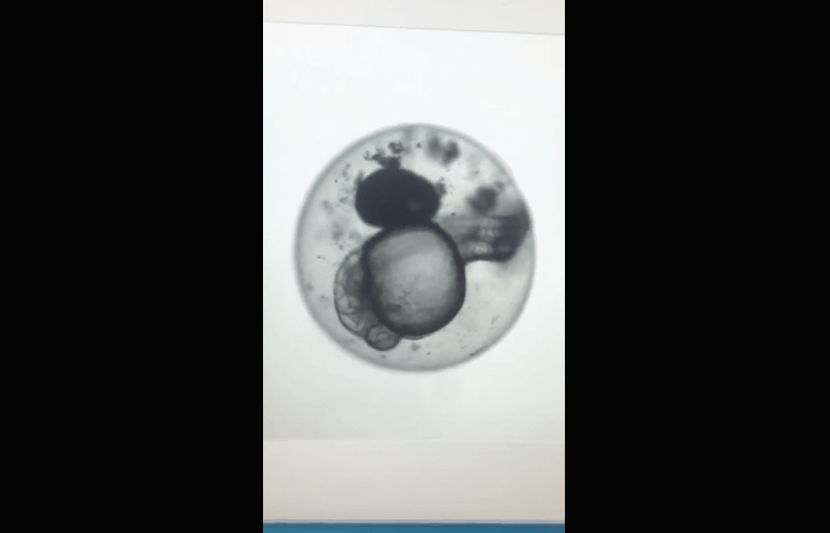
This new technology enabled the team to use a powerful beam of laser light to warm the embryo from -196°C to 20°C in just one-thousandth of a second.
“This rapid warming reduced the growth of deadly ice crystals that can grow during the freezing and rewarming process,” said Bischof. “Because the embryos were so large, we needed to warm them at millions of degrees per minute. Importantly, without this laser technique all embryos are non viable, but with the laser nanoparticle technique some of the embryos go on to develop out to 24 hours.”
The embryos that went on to mature to 24 hours developed a heart, gills, tail musculature and moved.
“There’s no doubt that the use of this technology, in this way, marks a paradigm shift for cryopreservation and the conservation of many wildlife species,” said Mary Hagedorn, an SCBI research scientist and paper co-author who has been working on cryopreserving zebrafish embryos since 1992. “Here we take a unique approach by combining biology with an exciting engineering technology to do what has been impossible previously: to successfully freeze and thaw a fish embryo so that the embryo begins to develop, rather than falls apart.”
This recent study has many beneficial implications for the future. Bischof is confident that, with further modification, the embryos will be able to fully grow into fish. They could then go on to reproduce and repopulate depleting colonies.
Because fish embryos are similar in shape and size to that of other marine and amphibious life, scientists can use this technology to cryopreserve eggs and embryos to support shrinking coral reef and frog populations.
Additionally, the results will have a positive effect on three areas of human needs and endeavours. “First, it will help preserve fish needed for food (through aquaculture), second it will help enhance our understanding human disease by supporting fish research models of human disease, and third it will help support conservation of endangered species,” Bischof told TUN.
Other lead authors in the study included University of Minnesota Ph.D. students Kanav Khosla and Yiru Wang. Additional help came from former University of Minnesota Ph.D. student Zhenpeng Qin.