Researchers from the University of Bristol’s School of Chemistry have successfully turned beer into sustainable gasoline using a new technology they developed. This brings them one step closer to converting ethanol, using beer as a model, into sustainable fuel on an industrial level.
A commonly accepted need for sustainable alternatives to fossil fuels for gasoline has caused researchers to utilize a number of substitutes, most commonly, bioethanol.
The U.S. and Brazil are the most notable examples of using bioethanol. In the U.S., gasoline is sold with a mix of up to 10 percent ethanol fuel blends.
However, ethanol is not a completely ideal alternative. It has a much lower energy density, mixes too easily with water, and can be fairly corrosive to engines.
For these reasons, butanol has been considered a much stronger alternative, but it has been difficult to make from sustainable sources.
Ethanol has previously been converted to butanol in laboratory conditions, using pure, dry ethanol, but in order for the technology to be scaled up, it needs to work with real ethanol fermentation broths.
The researchers had been contemplating these issues when they realized that the fermentation process necessary to make bioethanol looks a lot like the process of making beer.
“The alcohol in alcoholic drinks is actually ethanol – exactly the same molecule that we want to convert into butanol as a petrol replacement,” Professor Duncan Wass, whose team led the research, said in a statement.
Since bioethanol is made through essentially the same brewing process as beer — taking a sugar containing crop and fermenting it to make a mixture of ethanol (the “alcohol” in alcoholic drinks), water and some unfermented sugars and other molecules, the researchers discovered beer to be an ideal model to use for ethanol conversions.
“The only difference is that beer has hops added for flavouring,” Wass told The University Network (TUN).
So beer is the perfect model for an industrial ethanol fuel fermentation broth because it’s almost exactly the same.
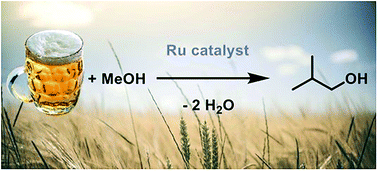
By using beer as a model for an ethanol mixture, the team has been able to work to convert the mixture into butanol by using catalysts they developed.
“All catalysts work by speeding up chemical reactions by offering an alternative reaction pathway which has a lower energy,” Wass told TUN.
“Our specific catalysts are based on ruthenium compounds (ruthenium is a fairly rare metal) that operate by converting two ethanol molecules (ethanol has two carbons) into one butanol molecule (butanol has four carbons) and some water. This chemical reaction would simply not happen without the catalyst.”
By demonstrating a successful conversion through using beer as a “real” ethanol mixture, the team has discovered a key step in scaling up this technology to larger application.
“There is still a lot to do to scale this up to an industrial process but demonstrating our technology works with this very close model for a real industrial source of ethanol is a very important step,” Wass told TUN.
“Using beer captures the imaginations but it has a serious point. It means bringing this sustainable fuel to market is closer than ever before.”